Principal Investigator
Philip Wedegaertner, PhD
Professor, Department of Biochemistry & Molecular Biology
Thomas Jefferson University
Team awards bring together basic, clinical, and population-based researchers to develop a collaboration. We particularly welcome multi-disciplinary teams of investigators that aim to produce preliminary data that will be competitive for a prestigious National Cancer Institute Program Project (PO1) Application and other research grants. The awards will be two years in duration and the second year of funding is contingent upon the team's research progress during the first 12 months.

Philip Wedegaertner, PhD
Professor, Department of Biochemistry & Molecular Biology
Thomas Jefferson University
This project addresses inhibition of mutant forms of the G proteins GNAQ and GNA11, early drivers of oncogenesis in more than 90% of ocular (uveal) melanomas, rare melanomas that occur in the eye and that frequently metastasize to the liver.
Metastatic uveal melanoma has been found to be resistant to targeted and immune therapies that have shown success in treating cutaneous melanoma, and there is a lack of effective therapies for metastatic uveal melanoma. Therefore, there is an unmet need for effective strategies to treat uveal melanoma. GNAQ and GNA11 are genes coding for highly similar proteins termed G protein alpha q and alpha 11. These G proteins are important regulators of cellular pathways that control cell proliferation. Their activity is normally tightly controlled by proteins called G protein-coupled receptors (GPCR) that allow the G protein to switch from an inactive state to an active state. However, in uveal melanoma GNAQ or GNA11 is frequently mutated at the amino acid glutamine 209 (Q209), resulting in mutant GNAQ or GNA11 being locked in the active state, thus driving uncontrolled cell proliferation. It is thought that the mutant GNAQ or GNA11 no longer relies upon GPCRs. However, we have discovered, surprisingly, that a GPCR termed HTR2B, which is highly expressed in uveal melanoma cells, plays a role in maintaining activation of mutant GNAQ. Treatment of uveal melanoma cells with inhibitors of HTR2B renders the cells more susceptible to inhibition by a known inhibitor of GNAQ/11 called YM-254890. The YM-254890 inhibitor has been well studied as a promising inhibitor of mutant GNAQ/11 in uveal melanoma. However, YM-254890 shows toxicity in mouse models at doses necessary for inhibition of uveal melanoma tumor growth.
Our project is focused on testing the proposal that inhibition of HTR2B in uveal melanoma will increase the ability of YM-254890 to block uveal melanoma cell growth, thereby providing a therapeutic window in which a lower dose of YM-254890 can inhibit tumor growth with decreased toxicity. Our research, focused on mutant GNAQ/GNA11, hopes to improve the treatment and quality of life of those suffering from metastatic uveal melanoma.

Co-Principal Investigator
Mizue Terai, PhD
Research Assistant Professor, Department of Medical Oncology
Thomas Jefferson University

Consultant
Jeffrey Benovic, PhD
Thomas Eakins Endowed Professor, Department of Biochemistry & Molecular Biology
Thomas Jefferson University

Haifeng Yang, PhD
Associate Professor, Department of Pathology and Genomic Medicine
Thomas Jefferson University
Uveal melanoma (UM) is the deadliest form of eye cancer in adults. Mutations in the BRCA-associated Protein 1 (BAP1) gene are strongly linked to a higher risk of metastasis. Once the cancer spreads, patient prognosis is poor and treatment options are limited. Current immunotherapies, including tebentafusp, have low response rates (3.6–18%) and do not provide long-lasting disease control. Although BAP1 loss is associated with slower growth of UM cells in the lab and greater stress tolerance, it is not known whether BAP1 mutations make these cells vulnerable to any treatment. This represents an urgent unmet medical need.
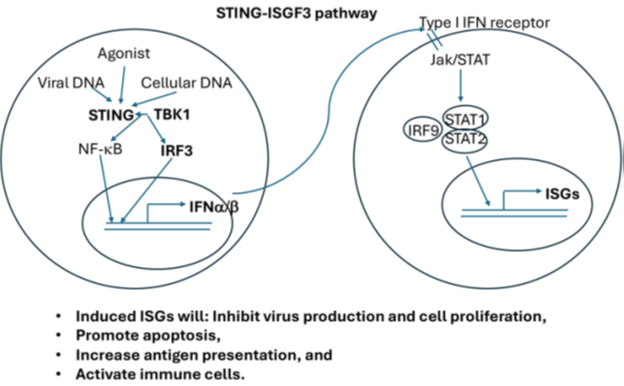
BAP1 mutations also occur frequently in clear cell renal cell carcinoma (ccRCC), a major type of kidney cancer. We found that loss of BAP1 in ccRCC cells, primary mouse kidney cells, mesothelial cells, and human mesothelioma cells all led to changes in the expression of Stimulator of Interferon Genes (STING), suggesting a strong connection between BAP1 and STING. The STING pathway is an important immune pathway that can influence cancer in both positive and negative ways. Activating this pathway can lead to cell death or reduced cell growth (Fig. 1). Based on this connection, we examined STING expression in biopsy samples from UM patients, which are mostly BAP1-deficient. We found that STING protein levels were highly elevated in all seventeen samples. We also observed that BAP1 loss in human UM tissue is associated with higher STING mRNA levels. In addition, a STING agonist showed strong effects against UM cell lines at low concentrations and worked even better when combined with the FDA-approved drug Bortezomib, a proteasome inhibitor. This combination killed UM cells quickly and almost completely within a day. Normal blood cells and many other cancer cell lines did not share this sensitivity, suggesting that this therapy is unlikely to harm most healthy cells.
We hypothesize that BAP1 loss in UM cells promotes STING activity and that pharmacological activation of STING can produce anti-tumor effects against UM. This will be investigated through the following aims:
Short-term goal: Find out if this combination can safely and effectively kill UM tumors in mice and understand why it works—paving the way for clinical trials.
Long-term goal: If successful, develop a new therapy for UM patients with liver metastases, improving survival and quality of life.
Fig. 1 Signaling Pathway of STING

Vitali Alexeev, PhD
Associate Professor, Department of Medical Oncology
Thomas Jefferson University

Sota Deguchi, MD, PhD
Postdoctoral Fellow, Department of Medical Oncology
Thomas Jefferson University

Mizue Terai, PhD
Research Assistant Professor, Department of Medical Oncology
Thomas Jefferson University

Claudia Capparelli, PhD
Assistant Professor, Department of Medical Oncology
Thomas Jefferson University
 This award was funded through a generous matching gift from The Ocular Melanoma Foundation, the Auburn Ocular Melanoma Group, and our patient families.
This award was funded through a generous matching gift from The Ocular Melanoma Foundation, the Auburn Ocular Melanoma Group, and our patient families.
Uveal melanoma (UM) is the most common cancer of the eye in adults. Unfortunately, half of UM patients develop advanced stage disease (metastasis) within 15 years of diagnosis, typically in the liver. Despite the recent FDA approval of a drug called Tebentafusp, there is still an urgent need for effective therapeutic strategies to treat late-stage UM, since this drug is only predicted to help a subset of UM patients. Standard chemotherapies rarely work in UM patients with large metastases, and only 30-50% of patients are alive one year later. Furthermore, UM does not respond well to targeted therapies, and clinical trials using immunotherapies only help a small number of patients. The lack of effective treatments for UM patients highlights the importance of identifying new therapeutic approaches to improve the treatment of UM patients.
In the proposed studies, we aim to explore the use of statins as a new therapeutic option to improve the response of UM to available therapies. A recent clinical trial (NCT03947385) indicated that the combination of two drugs, called darovasertib and crizotinib, may be a breakthrough treatment for UM, while statins are drugs that have been used for decades to lower cholesterol levels. Since statins are FDA-approved, generally well-tolerated, and preclinical studies indicate that these agents work together with targeted therapies to shrink tumors, we aim to investigate whether statins will improve response to darovasertib and crizotinib in UM. Additionally, given that statins have been shown to improve response to a type of immunotherapy, called PD-1 blocking antibodies, we will evaluate the effects of statins in combination with this immunotherapy in mice.
Our studies address the unmet need to optimize therapeutic strategies for UM patients. Our proposed research aims to investigate the effects of statins in combination with targeted and immune therapies in UM. At the end of this study, we aim to provide the scientific basis for more effective treatment options for this deadly form of melanoma.
Co-Principal Investigator
John Eisenbrey, PhD
Associate Professor, Department of Radiology
Thomas Jefferson University

Isabella Azzaro, MD
Research Assistant Intermediate, Department of Medical Oncology
Thomas Jefferson University

Nikita Thadani
Research Technician Intermediate, Department of Medical Oncology
Thomas Jefferson University

Corinne Wessner, MS, MBA, RDMS, RVT
Research Sonographer, Department of Radiology
Thomas Jefferson University

Isidore Rigoutsos, PhD, 2023 Ocular Melanoma Foundation Team Awardee
Richard W. Hevner Professor in Computational Medicine
Director, Computational Medicine Center, Thomas Jefferson University
Professor, Department of Pathology and Genomic Medicine, Department of Biochemistry & Molecular
Biology, and Department of Cancer Biology
 This award was funded through a generous matching gift from the Ocular Melanoma Foundation and our patient families.
This award was funded through a generous matching gift from the Ocular Melanoma Foundation and our patient families.
Melanoma of the eye (“uveal melanoma” – UM) is a rare tumor that originates from pigment-producing cells (melanocytes) in the eye. While UM is the most common primary eye malignancy in adults, it is rare compared to skin melanoma. Nearly one half of UM patients develop metastases that most often localize to the liver. Rapid tumor progression in the liver leads to death in 4 to 15 months. Metastatic UM (MUM) is highly resistant to chemotherapy. An alternative form of therapy called immunotherapy has also been explored in MUM. Immunotherapy activates the patient’s immune system to attack cancer cells in a targeted manner. While this approach has been very powerful in numerous contexts, and several drugs have already been approved by the US Food and Drug Administration, it has shown limited benefit for UM patients.
A new FDA-approved therapy for UM, Tebentafusp-tebn, is shown more promise. The therapy works by increasing the numbers of cancer-fighting cells and is currently the preferred treatment for 50% of Caucasians with MUM. Survival in patients receiving Tebentafusp-tebn was prolonged by approximately 6 months compared to standard care treatments. Response to treatment is typically evaluated through imaging. Imaging has its own challenges because of confounding factors. On the other hand, molecular markers that can gauge sensitively and specifically the response to treatment, and the speed of that response, could drastically improve the quality and speed of clinical decision making. In this project, we will explore a novel approach: we will develop biomarkers of response to treatment that are based on short non-coding RNAs (sncRNAs).
SncRNAs are important because they regulate the abundance of proteins, and, by extension, cell fate. Indeed, we now know that sncRNAs control numerous essential cellular processes, in health and disease. Advances during the last decade have demonstrated that the manner in which sncRNAs control proteins depends on tissue type and disease type. Thus, sncRNAs are very good candidates to serve as sensitive and specific diagnostic and prognostic tools, and this is why we chose them as the basis for this project.
The project has two parts. First, we will study liver tumors from MUM patients participating in a clinical trial at Jefferson. In these tumors, we will examine the levels of sncRNAs and of the RNA templates from which proteins are made. We will determine how these levels differ from what is seen in the primary tumors of UM patients who developed or did not develop metastases. We will determine how these levels change over time during treatment. Second, we will study sncRNAs in the blood of the same patients at different time points during treatment and determine how their levels differ from those in the blood of UM patients without metastases. We will determine how sncRNA blood levels change as treatment progresses and build a mathematical model to capture the speed of response to therapy. Once built, the model will allow us to accurately assess the response of patients while they are receiving the therapy.
Our short-term goal is to create biomarkers that leverage the power of sncRNAs in the MUM context. If successful, we expect to use the biomarkers in clinical settings to assist in clinical decision-making. Our long-term goal is to extend patient survival by creating novel targeted therapies for MUM. It is important to note that the methods we will develop in this project are very general in nature and can be used with other therapeutic regimens, and other cancers, where it is important to have molecular tests that accurately determine response to therapy in real-time.

Eric Londin, PhD
Assistant Professor
Department of Pathology, Anatomy and Cellular Biology

Marlana Orloff, MD
Alexander and Johnston Family Endowed Clinical Director of Uveal Melanoma
Associate Professor, Department of Medical Oncology

Mizue Terai, PhD
Research Assistant Professor
Department of Medical Oncology
Thomas Jefferson University

Mizue Terai, PhD
Research Assistant Professor
Department of Medical Oncology
Thomas Jefferson University
Uveal melanoma (UM) is a rare eye tumor that originates from pigment-producing cells (melanocytes) in the eye. Although rare compared to skin melanoma, it is the most common primary intraocular malignancy in adults. Over the past decade, substantial progress has been achieved in the treatment of primary eye lesions while preserving the affected eye. Nevertheless, up to 50% of UM patients subsequently develop systemic spread (metastasis) after successful therapy of the primary tumor in the eye. Once UM cells escape from the eye, about 80-90% of them spread to the liver where tumors rapidly progress, and patients die within four to 15 months. Metastatic UM (MUM) is highly resistant to chemotherapy and other tumor inhibitors. Therefore, there is still an unmet need for better treatment for MUM patients.
Immunotherapy utilizes the body’s immune system to recognize and destroy cancer cells. In the past decade, immunotherapy has become a powerful approach in treating various cancers including metastatic skin melanoma. However, some cancer cells create an immune-suppressive environment by either reducing the expression of antigens on their cell surface recognized by immune cells or by other mechanisms that escape the attack by the immune system.
Adoptive Cell Therapy (ACT) is a new form of immunotherapy that uses human or genetically engineered immune cells to eliminate cancer. ACT is a promising approach, especially for solid tumors, like MUM, which have shown low response to other therapies. ACT has been approved by the Food and Drug Administration (FDA) to treat hematologic (blood) malignancies. Natural killer (NK) cells are one of the key components in the immune system and are an ideal candidate for ACT, because MUM cells tend to express molecules recognized by NK cells. To overcome the technical challenge of conventional NK cell therapy, which is the limitation of NK cells obtained from human or cell lines, we collaborated with HEALIOS K.K. from Japan, one of the leaders in engineered stem cell (iPSC, induced pluripotent blood cells) therapy. iPSC benefits include genetic engineering, increased expression of cytokines and chemokines and their receptors, and a loweredrisk of infection and rejection, because there are no donor cell requirements. We will explore the potential of iPSC-derived NK cells in the treatment of MUM.
To achieve the goal, we proposed the following two projects in this team research award application:
Aim 1: Investigate the anti-tumor activity of iPSC-NK cells against MUM cells. To investigate the activities of iPSC-NK cells against MUM cells, at least three MUM cell lines will be employed to study the killing activities and the production of immune stimulants by iPSC-NK cells. In addition, we will investigate the migration of iPSC-NK cells toward MUM cells.
Aim 2: Investigate in vivo activity of iPSC-NK cells in MUM mouse models. This aim is to test the accumulation and killing activity of iPSC-NK cells against MUM in the MUM metastasis mouse model. We have already established a MUM liver metastatic mouse model using NSG mice without mouse immune cells. We will investigate MUM tumor growth suppression by iPSC-NK cells in mouse models.
Our future goal of this project is to conduct a clinical trial of liver direct infusion therapy for MUM patients using immune cells, such as NK cells, to eliminate MUM effectively. Our overall goal is to completely eradicate MUM cells in patients with MUM.

Vitali Alexeev, PhD
Research Assistant Professor
Departments of Dermatology and Medical Oncology
Thomas Jefferson University

Sota Deguchi, MD, PhD
Postdoctoral Fellow
Department of Medical Oncology
Thomas Jefferson University

Yuri Sykulev, MD, PhD
Professor, Department of Microbiology & Immunology
Thomas Jefferson University

Isidore Rigoutsos, PhD, 2023 Ocular Melanoma Foundation Team Awardee
Richard W. Hevner Professor in Computational Medicine
Director, Computational Medicine Center, Thomas Jefferson University
Professor, Department of Pathology and Genomic Medicine, Department of Biochemistry & Molecular
Biology, and Department of Cancer Biology
 This award was funded through a generous matching gift from the Ocular Melanoma Foundation and our patient families.
This award was funded through a generous matching gift from the Ocular Melanoma Foundation and our patient families.
Melanoma of the eye (“uveal melanoma” – UM) is a rare tumor that originates from pigment-producing cells (melanocytes) in the eye. While UM is the most common primary eye malignancy in adults, it is rare compared to skin melanoma. Nearly one half of UM patients develop metastases that most often localize to the liver. Rapid tumor progression in the liver leads to death in 4 to 15 months. Metastatic UM (MUM) is highly resistant to chemotherapy. An alternative form of therapy called immunotherapy has also been explored in MUM. Immunotherapy activates the patient’s immune system to attack cancer cells in a targeted manner. While this approach has been very powerful in numerous contexts, and several drugs have already been approved by the US Food and Drug Administration, it has shown limited benefit for UM patients.
A new FDA-approved therapy for UM, Tebentafusp-tebn, is shown more promise. The therapy works by increasing the numbers of cancer-fighting cells and is currently the preferred treatment for 50% of Caucasians with MUM. Survival in patients receiving Tebentafusp-tebn was prolonged by approximately 6 months compared to standard care treatments. Response to treatment is typically evaluated through imaging. Imaging has its own challenges because of confounding factors. On the other hand, molecular markers that can gauge sensitively and specifically the response to treatment, and the speed of that response, could drastically improve the quality and speed of clinical decision making. In this project, we will explore a novel approach: we will develop biomarkers of response to treatment that are based on short non-coding RNAs (sncRNAs).
SncRNAs are important because they regulate the abundance of proteins, and, by extension, cell fate. Indeed, we now know that sncRNAs control numerous essential cellular processes, in health and disease. Advances during the last decade have demonstrated that the manner in which sncRNAs control proteins depends on tissue type and disease type. Thus, sncRNAs are very good candidates to serve as sensitive and specific diagnostic and prognostic tools, and this is why we chose them as the basis for this project.
The project has two parts. First, we will study liver tumors from MUM patients participating in a clinical trial at Jefferson. In these tumors, we will examine the levels of sncRNAs and of the RNA templates from which proteins are made. We will determine how these levels differ from what is seen in the primary tumors of UM patients who developed or did not develop metastases. We will determine how these levels change over time during treatment. Second, we will study sncRNAs in the blood of the same patients at different time points during treatment and determine how their levels differ from those in the blood of UM patients without metastases. We will determine how sncRNA blood levels change as treatment progresses and build a mathematical model to capture the speed of response to therapy. Once built, the model will allow us to accurately assess the response of patients while they are receiving the therapy.
Our short-term goal is to create biomarkers that leverage the power of sncRNAs in the MUM context. If successful, we expect to use the biomarkers in clinical settings to assist in clinical decision-making. Our long-term goal is to extend patient survival by creating novel targeted therapies for MUM. It is important to note that the methods we will develop in this project are very general in nature and can be used with other therapeutic regimens, and other cancers, where it is important to have molecular tests that accurately determine response to therapy in real-time.

Yuri Sykulev, MD, PhD
Professor, Department of Microbiology & Immunology
Thomas Jefferson University
Yuri Sykulev, MD, PhD, is leading a team with a focus on developing effective T cell therapeutics for the treatment of metastatic uveal melanoma (UM). T cell therapies have shown promising responses in multiple refractory solid tumors including skin melanoma. Thus far, the most promising T cell therapy to treat skin melanoma is infusion of tumor infiltrating lymphocytes (TIL), which recognize tumor-specific neo-antigens abundant in skin melanoma. The barrier to successful treatment of UM with TIL is much lower frequency of tumor-reactive T cells within the metastases and strength of their anti-tumor response. To overcome the barriers for production of highly effective UM-specific T cell therapeutics, researchers propose to utilize TCR genes that recognize driver mutations of UM (GNAQ/GNA11 mutations). The researchers design to incorporate these TCR genes to otherwise non-specific T cells to manufacture UM-specific TCR-T cells to produce “off-shelf” products. The research team will investigate various strategies to generate these melanoma-specific TCR-T cells and will compare the ability of these cells to control the growth of human UM cells in vitro and in mouse models. The goal of this multi-phased project is to begin the development of effective T cell therapeutics, which would change the treatment for future uveal melanoma patients.
It is becoming increasingly evident that people have differential abilities to control viral infections and transform normal tissues that lead to cancer development. Analysis of immune responses revealed that CD8 T cells play an essential role in controlling viruses and cancer. Various experimental approaches provided evidence that the ability of CD8 T cells to destroy aberrant cells could vary widely among people.
Indeed, analysis of killing potency of CD8 T cells derived from people with no symptoms of a viral infection demonstrated powerful CD8 T cells responses against target cells presenting viral proteins in in vitro assays. People capable of controlling viral infections are often called “elite controllers”. Most likely, elite controllers inherited a unique combination of genes encoding T-cell receptors on CD8 T cells that can induce a unique response to destroy aberrant cells, either virally infected or transformed cells.
These considerations informed our approach to replace available genes encoding TCR in every T cell with the TCR genes identified in uveal melanoma “elite controllers”. Expanded in vitro TCR-T cells will be introduced into patients to initiate a potent response against aberrant cells. We propose to apply the above approach to treat very rare aggressive Uveal melanoma (UM).
The median survival rate after the onset of metastases is 6 months. Findings of Dr. Takami Sato and colleagues indicate that some patients control metastases significantly longer than others, suggesting that these patients might have more potent CD8 T cells. Our approach utilizes available TCR genes in CD8 T cells of people whose CD8 T cells are less potent with the genes derived from patients capable of controlling the metastases significantly longer or, perhaps, eliminating the metastases at all. Potency of the ability of the two kinds of CD8 T cells, natural and engineered, will be compared in in vitro cytolytic assay hoping to detect more sensitive response of engineered TCR-T cells. If so, these engineered T cells will be tested in their ability to control UM metastasis in humanized mice.

Vitali Alexeev, PhD
Research Assistant Professor
Departments of Dermatology and Medical Oncology
Thomas Jefferson University

Nadia Anikeyeva, PhD
Research Instructor
Department of Microbiology and Immunology
Thomas Jefferson University

Mizue Terai, PhD
Research Assistant Professor
Department of Medical Oncology
Thomas Jefferson University

Gyorgy Hajnoczky, MD, PhD
Director, Mitochondrial Research Center
Professor, Thomas Jefferson University
Gyorgy Hajnoczky, MD, PhD, is leading a team with a focus on mitochondrial diagnostics and therapy to investigate the development of novel therapeutics for uveal melanoma. This research team brings together expertise in uveal melanoma liver metastases (Mizue Terai, PhD, Assistant Professor, Thomas Jefferson University), the inherited defect (Philip B. Wedegaertner, PhD, Professor, Thomas Jefferson University), and therapeutic cell death activation (Gyorgy Hajnoczky, MD, PhD, Professor, Thomas Jefferson University) to test this hypothesis and understand the disease process and therapy of uveal melanoma. Piyush Mishra, PhD, a senior fellow, is the primary driving force in moving this uveal melanoma project further.
The group discovered that primary liver cancer and normal liver have distinctive cell death signatures, and developed an approach based on this difference to kill hepatocarcinoma without harming normal liver. Because uveal melanoma mortality is primarily due to its liver metastases, they speculated whether the liver metastases are also different from normal liver in their cell death signature and can be selectively targeted with the therapy that is effective for primary liver cancer. This project has the opportunity to develop new therapeutics for this disease, which would have an immeasurable impact on metastatic uveal melanoma patients.
Nearly all uveal melanoma patients have an inherited problem in a factor controlling communication by calcium in the cells. Through this project, we have demonstrated that the patients’ cells show spontaneous calcium oscillations. We have established new genetic models to test the relevance of this in hepatic metastases of uveal melanoma.
The group discovered that primary liver cancer and a normal liver have distinctive cell death signatures, and developed an approach based on this difference to kill hepatocarcinoma without harming a normal liver (Nature Communications 2024 in press). We have created a combination therapy approach that selectively kills tumor cells in uveal melanoma liver metastases in vitro.
All in all, our project created an opportunity for several outstanding investigators with complementary expertise to come together to make a difference in the understanding of the disease process and therapy of uveal melanoma.

Sergei Koshkin, PhD
Postdoctoral Research Fellow
Department of Medical Oncology
Sidney Kimmel Cancer Center
Thomas Jefferson University

Jiansong Luo, PhD
Research Associate
Biochemistry & Molecular Biology
Thomas Jefferson University

Piyush Mishra, PhD
Postdoctoral Research Fellow
Pathology
Thomas Jefferson University

Mizue Terai, PhD
Research Assistant Professor
Department of Medical Oncology
Thomas Jefferson University

Philip Wedegaertner, PhD
Professor, Department of Biochemistry & Molecular Biology
Thomas Jefferson University
2022 Team Awardees now include two-year progress summaries.
Mitchell Berkowitz, BA
Program Manager, Melanoma Research Institute of Excellence
IIT Development Coordinator
Department of Medical Oncology
mitchell.berkowitz@jefferson.edu
215-955-8745
Elena Boroski
Director of Development, Sidney Kimmel Cancer Center
Office of Institutional Advancement
elena.boroski@jefferson.edu
267-225-8380